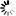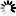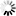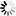PROTEOSTAT(R) 蛋白聚集检测
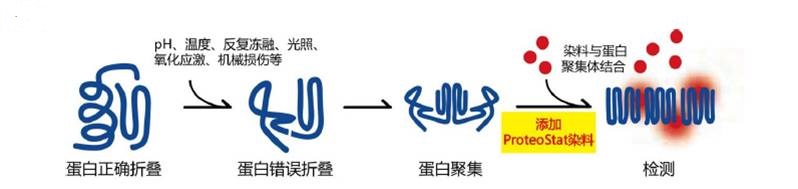
|
案例一
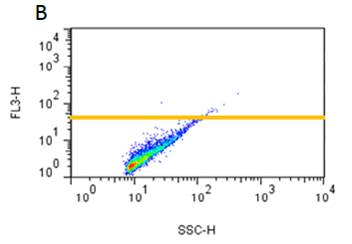
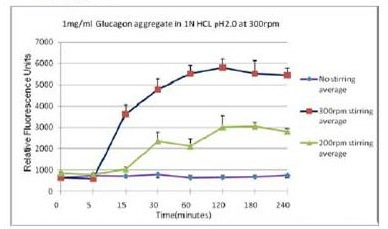 不同搅拌速率对蛋白聚集的影响
|
案例二
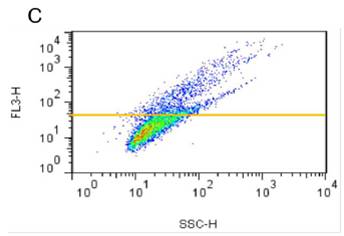
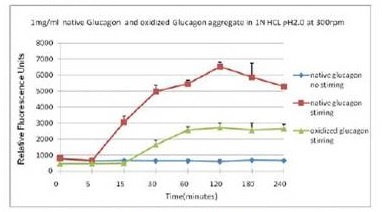 蛋氨酸被氧化后可抑制蛋白聚集
|
|
使用本产品检测后,可知搅拌速度越快,蛋白聚集程度越大。
|
蓝色曲线为对照,没有搅拌速度在 300 rpm 下,绿色曲线与红色曲线分别表示蛋氨酸被氧化后的蛋白聚集情况与天然蛋白的聚集情况。使用本产品检测后,可知蛋氨酸被氧化后可抑制蛋白聚集。
|
该试剂盒专门设计用于监测溶液中蛋白质聚集体的形成。
ENZ-51023-KP050:在96孔板中进行50次测试,或流式细胞仪测试16次
ENZ-51023-KP002: 96孔测试2次或流式细胞仪测试70次
- Exposure of α-Synuclein Aggregates to Organotypic Slice Cultures Recapitulates Key Molecular Features of Parkinson's Disease: S. Moudio, et al.; Front. Neurol. 13, 826102 (2022);
- GrpEL1 regulates mitochondrial unfolded protein response after experimental subarachnoid hemorrhage in vivo and in vitro: C. Ma, et al.; Brain Res. Bull. 181, 97 (2022);
- Identification of an endoplasmic reticulum proteostasis modulator that enhances insulin production in pancreatic β cells: M. Miyake, et al.; Cell Chem. Biol. 1016, S2451 (2022);
- Protocol for isolation and proteostatic analysis of sub-populations of spermatogenic cells in mouse: Q. Zou, et al.; STAR Protoc. 3, 101398 (2022);
- ATP-citrate lyase promotes axonal transport across species: A. Even, et al.; Nat. Commun. 12, 5878 (2021);
- Cell-Instructive Surface Gradients of Photoresponsive Amyloid-like Fibrils: A.M. Bender, et al.; ACS Biomater. Sci. Eng. 7, 4798 (2021);
- Proteostasis regulated by testis-specific ribosomal protein RPL39L maintains mouse spermatogenesis: Q. Zou, et al.; iScience 24, 103396 (2021);
- Rejuvenation of tumour-specific T cells through bispecific antibodies targeting PD-L1 on dendritic cells: L. Liu, et al.; Nat. Biomed. Eng. 5, 1261 (2021);
- Characterization of Protein Aggregates, Silicone Oil Droplets, and Protein-Silicone Interactions using Imaging Flow Cytometry: C. Probst; J. Pharm. Sci. 109, 364 (2020);
- Endoplasmic reticulum chaperone calmegin is upregulated in aldosterone-producing adenoma and associates with aldosterone production: K. Itcho, et al.; Hypertension 75, 492 (2020);
- Monitoring plasma protein aggregation during aging using conformation-specific antibodies and FTIR spectroscopy: S. Magalhaes, et al.; Clin. Chim. Acta 502, 25 (2020), Application(s): Protein aggregation in human plasma samples;
- PERK participates in cardiac valve development via fatty acid oxidation and endocardial-mesenchymal transformation: T. Shimizu, et al.; Sci. Rep. 10, 20094 (2020);
- The polyphenol quercetin protects from glucotoxicity depending on the aggresome in Caenorhabditis elegans: M. Civelek, et al.; Eur. J. Nutr. 59, 485 (2020), Application(s): Protein aggregation in solution;
- Fibril formation and therapeutic targeting of amyloid-like structures in a yeast model of adenine accumulation: D. Laor, et al.; Nat. Commun. 10, 62 (2019);
- Transthyretin amyloid fibril disrupting activities of extracts and fractions from Juglans mandshurica maxim. var. cordiformis (Makino) kitam: N. Chaudhary, et al.; Molecules 24, 500 (2019), Application(s): Aged amyloid fibrils;
- Caffeic acid and resveratrol ameliorate cellular damage in cell and Drosophila models of spinocerebellar ataxia type 3 through upregulation of Nrf2 pathway: Y.L. Wu, et al.; Free Radic. Biol. Med. 115, 309 (2018);
- Discovering putative prion-like proteins in Plasmodium falciparum: A computational and experimental analysis: I. Pallares, et al.; Front. Microbiol. 9, 1737 (2018), Application(s): P. falciparum culture;
- High Throughput Differential Scanning Fluorimetry (DSF) Formulation Screening with Complementary Dyes to Assess Protein Unfolding and Aggregation in Presence of Surfactants: S.M. McClure, et al.; Pharm. Res. 35, 81 (2018);
- The catalytic inactivation of the N-half of human hexokinase 2 and structural and biochemical characterization of its mitochondrial conformation: M.H. Nawaz, et al.; BioSci. Rep. 38, BSR20171666 (2018), Application(s): Aggregation propensity assessed by DSF;
- Aggregated transthyretin is specifically packaged into placental nano-vesicles in preeclampsia: M. Tong, et al.; Sci. Rep. 7, 6694 (2017), Application(s): Protein aggregation in extracts from placental extracellular vesicles;
- Curcumin Improves Palmitate-Induced Insulin Resistance in Human Umbilical Vein Endothelial Cells by Maintaining Proteostasis in Endoplasmic Reticulum: M. Ye, et al.; Front. Pharmacol. 8, 148 (2017);
- Scaffold requirements for periodontal regeneration with enamel matrix derivative proteins: A. Apicella, et al.; Colloids Surf. B Biointerfaces 156, 221 (2017), Application(s): Aggregation of enaml matrix derivative (EMD) proteins;
- Trans ε-viniferin is an amyloid-β disaggregating and anti-inflammatory drug in a mouse primary cellular model of Alzheimer's disease: E. Vion, et al.; Mol. Cell. Neurosci. 88, 1 (2017); ARD1-mediated Hsp70 acetylation balances stress-induced protein refolding and degradation: J.H. Seo, et al.; Nat. Commun. 7, 12882 (2016), Application(s): ;
- BRG1 and BRM SWI/SNF ATPases redundantly maintain cardiomyocyte homeostasis by regulating cardiomyocyte mitophagy and mitochondrial dynamics in vivo: S.J. Bultman, et al.; Cardiovasc. Pathol. 25, 258 (2016);
- Cardiomyocyte-specific human Bcl2-associated anthanogene 3 P209L expression induces mitochondrial fragmentation, Bcl2-associated anthanogene 3: M.T. Quintana, et al.; Am. J. Pathol. 186, 1989 (2016), Application(s): Lysates from cardiac tissues;
- Interfacial dilatational deformation accelerates particle formation in monoclonal antibody solutions: G.L. Lin, et al.; Soft Matter 12, 3293 (2016);
- Resveratrol reduces amyloid-beta (Aβ1–42)-induced paralysis through targeting proteostasis in an Alzheimer model of Caenorhabditis elegans: C. Regitz, et al.; Eur. J. Nutr. 55, 741 (2016), Application(s): Protein aggregation in solution;
- A screening methodology for purifying proteins with aggregation problems: M. Lebendiker, et al.; Methods Mol. Biol. 1258, 261 (2015);
- Effects of tau domain-specific antibodies and intravenous immunoglobulin on tau aggregation and aggregate degradation: J.O. Esteves-Villanueva, et al.; Biochemistry 54, 293 (2015);
- Metal-mediated protein oxidation: applications of a modified ELISA-based carbonyl detection assay for complex proteins: H. Uehara, et al.; Pharm. Res. 32, 691 (2015), Application(s): Effects of metal-mediated protein oxidation on aggregation;
- WALTZ-DB: a benchmark database of amyloidogenic hexapeptides: J. Beerten, et al.; Bioinformatics 31, 1698 (2015);
- Amyloid-beta (Aβ1-42)-induced paralysis in Caenorhabditis elegans is reduced by restricted cholesterol supply: C. Regitz, et al.; Neurosci. Lett. 576, 93 (2014);
- Human Stefin B Role in Cell's Response to Misfolded Proteins and Autophagy: M. Polajnar, et al.; PLoS One 9, e102500 (2014), Application(s): Proteins aggregation and oxidative stress in stefin B KO astrocytes;
- Lysosomal enzyme cathepsin B enhances the aggregate forming activity of exogenous α-synuclein fibrils: A. Tsujimura, et al.; Neurobiol. Dis. 73C, 244 (2014), Application(s): In vitro formation of a-synuclein fibrils and monitoring with ProteoStat? dye;
- ProteoStat to detect and discriminate intracellular amyloid-like aggregates in Escherichia coli: S.Navarro & S.Ventura; Biotechnol. J 9, 1259 (2014);
- Increased carbonylation, protein aggregation and apoptosis in the spinal cord of mice with experimental autoimmune encephalomyelitis: A. Dasgupta, et al.; ASN Neuro 5, e00111 (2013), Application(s): Detection of protein aggregation in tissue section using fluorescence microscopy;
- Optimization of protein purification and characterization using Thermofluor screens: S. Boivin, et al.; Protein Expr. Pur. 91, 192 (2013);
- Protein quality control acts on folding intermediates to shape the effects of mutations on organismal fitness: S. Bershtein, et al.; Mol. Cell. 49, 133 (2013), Application(s): Propensity to aggregate for dihydrofolate reductase mutants;
- Correction of both NBD1 energetics and domain interface is required to restore ΔF508 CFTR folding and function: W.M. Rabeh, et al.; Cell 148, 150 (2012), Application(s): Aggregation of multi-domain protein: NBD1;
- Detection of α-synuclein amyloidogenic aggregates in vitro and in cells using light-switching dipyridophenazine ruthenium(II) complexes: N.P. Cook, et al.; J. Am. Chem. Soc. 134, 20776 (2012);
- p62/SQSTM1-Dependent Autophagy of Lewy Body-Like α-Synuclein Inclusions: Y. Watanabe, et al.; PLoS One 7, e52868 (2012), Application(s): Protein aggregation of alpha-synuclein;
- Raster image correlation spectroscopy as a novel tool for the quantitative assessment of protein diffusional behaviour in solution: Z. Hamrang, et al.; J. Pharm. Sci. 101, 2082 (2012);
- High sensitivity luminescence nanoparticle assay for the detection of protein aggregation: S. Pihlasalo, et al.; Anal. Chem. 83, 1163 (2011);
 |
 |
 |
| 产品编号 | 产品名称 | 产品规格 |
| ENZ-51023-KP050 | PROTEOSTAT® 蛋白聚集检测 PROTEOSTAT® Protein aggregation assay |
50 tests |
| ENZ-51023-KP002 | 2×96 tests |
|
产品编号
|
产品名称
|
包装
|
|
ENZ-51039-KP002
|
PROTEOSTAT® 蛋白质聚集标准品套装
PROTEOSTAT® Protein aggregation standards |
2x96 wells
|
 人IgG多抗
人IgG多抗|
浓度
|
1mg/ml
|
|
来源
|
兔
|
|
免疫原
|
人免疫球蛋白G
|
|
物种反应性
|
人
|
|
应用
|
WB
|
|
纯化细节
|
蛋白A亲和纯化
|
|
形式
|
液体。溶于含有10ppm卡松防腐剂的PBS缓冲液中。
|
|
使用/稳定性
|
在4°C下可稳定1个月,在-20°C下可稳定至少12个月。
|
|
处理
|
避免反复冻融
|
|
运输
|
冰袋运输
|
|
短期储存
|
+4°C
|
|
长期储存
|
-20°C
|
|
监管状态
|
RUO - 仅供研究使用
|
|
产品编号
|
产品名称
|
包装
|
|
ENZ-ABS264-1000
|
人IgG多抗
IgG (human) polyclonal antibody |
1 ml
|
 |
 |
 |
| 官网:www.cxbio.com | 微信服务号:iseebio | 微博:seebiobiotech |
 |
 |
 |
| 商城:mall.seebio.cn | 微信订阅号:seebiotech | 泉养堂:www.canmedo.com |
相关资讯
- Cell Rep:突破!科学家阐明单个线粒体的首个DNA序列
- Nature揭示:植物如何在严寒酷暑、害虫啃食下求生
- 有关工业4.0的三个神话与一个真相
- IL-33 (人), ELISA 试剂盒 IL-33 (human), ELISA kit
- 生物防御抗原(Ag)、抗体(Ab)、酶
- 疾病诊断及研究用试剂盒优惠促销
- 试剂盒的性能可能有问题,结果与预期不一致!我该怎么办?
- Science:首次从结构上揭示帕金森病的关键组分的毒性产生机制
- N-聚糖标准品 - Ludger糖基化产品
- 胃蛋白酶原II(PGII)-磁微粒化学发光法(AE/AP)/荧光免疫层析解决方案(含保护剂评估)
新进产品
同类文章排行
- LBIS(R)小鼠卵清蛋白特异性免疫球蛋白G1(OVA-IgG1)ELISA试剂盒
- LBIS(R)小鼠卵清蛋白特异性免疫球蛋白G1(OVA-IgG1)ELISA试剂盒
- LBIS(R) 血蓝蛋白(KLH)(T细胞依赖性抗原) 大鼠免疫球蛋白M(IgM) ELISA试剂盒
- 大鼠免疫球蛋白G(IgG)ELISA试剂盒
- 疫苗佐剂:疫苗效力的隐形推手
- “小标记”“大变身” - 新升级辣根过氧化物酶(HRP)
- LBIS(R) Mouse MCP-1(CCL-2) ELISA Kit
- Apelin中和抗体 Anti Apelin, Monoclonal Antibody(4G5)
- 搭建细菌培养温床的“混凝土和复合肥” - 琼脂粉探秘
- LBIS(R) Human Apo B-48 ELISA Kit 人血清载脂蛋白 B-48(ApoB-48)ELISA 试剂盒
资讯文章
您的浏览历史