糖基化反应中的冉冉新星——蔗糖磷酸化酶
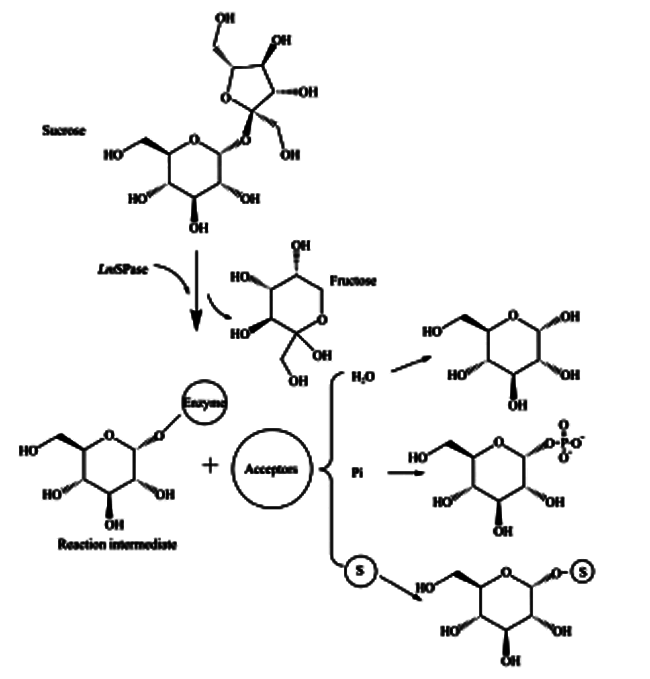
SPase催化的反应图

|
酶
Enzymes
|
反应条件
Reaction conditions
|
产量
Yield
|
参考文献
References
|
|
LmSP
|
40 g/L hydroquinone, sucrose: hydroquinone=5:1, pH 7.0, 30 ℃, 24 h
|
98.00 g/L
|
[24]
|
|
SmSPI336L
|
Whole cell transformation
|
110.30 g/L
|
[25]
|
|
Amylosucrase
|
20 mmol/L sucrose, 5?mmol/L hydroquinone, pH 7.0, 40 ℃
|
44.70%
|
[26]
|
|
XcAS
|
1 mol/L sucrose, 200 mmol/L hydroquinone
|
53.86 g/L
|
[27]
|
|
Dextransucrase
|
215 mmol/L sucrose, 450 mmol/L hydroquinone
|
544.00 mg/L
|
[28]
|
|
酶
Enzymes
|
反应条件
Reaction conditions
|
产量
Yield
|
参考文献
References
|
|
LmSP
|
0.8 mol/L sucrose, 2.0 mol/L glycerol, 20 U/mL
SPase, pH 7.0, 30 ℃
|
63.0%
|
[31]
|
|
LmSP
|
Immobilized Z
|
85.0%
|
[32]
|
|
LmSP
|
Whole-cell catalyst, 1 mol/L sucrose, 2.5 mol/L glycerol, pH 7.0, 30 ℃
|
189.3 g/L
|
[33]
|
|
BaSP/L341W
|
200 g/L sucrose, 200 g/L glycerol, 30 ℃
|
86.0 g/L
|
[29]
|
|
货号
|
产品名称
|
规格
|
|
ECE1080B
|
Seebio® 蔗糖磷酸化酶(Sucrose Phosphorylase)
|
1ku
|
|
ECE1080C
|
Seebio® 蔗糖磷酸化酶2.0
|
1ku
|
|
产品名称
|
规格
|
纯度
|
2-α-GG content含量
|
|
甘油葡萄糖苷(a-GG)
|
1kg
|
>90%
|
40-55%
|
[2] YJiang R N, Ye K, Gan T, et al. Application of sucrose phosphorylase in glycosylation. Chinese Journal of Biotechnology, 2021, 37(1): 112-129.
[3] Kiessling LL, Splain RA. Chemical approaches to glycobiology[J]. Annual Review of Biochemistry, 2010, 79: 619-653. DOI:10.1146/annurev.biochem.77.070606.100917
[4] Lindhorst TK. Essentials of carbohydrate chemistry and biochemistry[J]. Carbohydrate Polymers, 2002, 47(1): 87
[5] De Roode BM, Franssen MCR, Van Der Padt A, et al. Perspectives for the industrial enzymatic production of glycosides. Biotechnol Prog, 2003, 19(5): 1391-1402. DOI:10.1021/bp030038q
[6] Lombard V, Ramulu HG, Drula E, et al. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res, 2014, 42(D1): D490-D495. DOI:10.1093/nar/gkt1178
[7] Goedl C, Sawangwan T, Wildberger P, et al. Sucrose phosphorylase: a powerful transglucosylation catalyst for synthesis of α-D-glucosides as industrial fine chemicals. Biocatal Biotransfor, 2010, 28(1): 10-21. DOI:10.3109/10242420903411595
[8] Kagan BO, Latker SN, Zfasman EM. Phosphorolysis of saccharose by cultures of Leuconostoc mesenteroides[J]. Biokhimiya, 1942, 7: 93-108.
[9] Hou GW, Ma JF, Sui SS, Jiang M, Wei P. Research progress on production and application of sucrose phosphorylase[J]. China Brewing, 2010, 29(6): 17-20. (in Chinese)
[10] Russell RR, Mukasa H, Shimamura A, Ferretti JJ. Streptococcus mutans gtfA gene specifies sucrose phosphorylase[J]. Infection and Immunity, 1988, 56(10): 2763-2765. DOI:10.1128/iai.56.10.2763-2765.1988
[11] Desmet T, Soetaert W. Enzymatic glycosyl transfer: mechanisms and applications[J]. Biocatalysis and Biotransformation, 2011, 29(1): 1-18. DOI:10.3109/10242422.2010.548557
[12] Schwarz A, Nidetzky B. Asp-196→Ala mutant of Leuconostoc mesenteroides sucrose phosphorylase exhibits altered stereochemical course and kinetic mechanism of glycosyl transfer to and from phosphate. FEBS Lett, 2006, 580(16): 3905-3910. DOI:10.1016/j.febslet.2006.06.020
[13] Mueller M, Nidetzky B. The role of Asp-295 in the catalytic mechanism of Leuconostoc mesenteroides sucrose phosphorylase probed with site-directed mutagenesis. FEBS Lett, 2007, 581(7): 1403-1408. DOI:10.1016/j.febslet.2007.02.060
[14] Wiesbauer J, Goedl C, Schwarz A, et al. Substitution of the catalytic acid-base Glu237 by Gln suppresses hydrolysis during glucosylation of phenolic acceptors catalyzed by Leuconostoc mesenteroides sucrose phosphorylase. J Mol Catal B: Enzym, 2010, 65(1/4): 24-29.
[15] Wang MM, Wu J, Wu D. Cloning and expression of the sucrose phosphorylase gene in Bacillus subtilis and synthesis of kojibiose using the recombinant enzyme[J]. Microbial Cell Factories, 2018, 17(1): 23. DOI:10.1186/s12934-017-0842-2
[16] Lin JF, Xie L, Guo LQ, Ye ZW. Application of recombinant sucrose phosphatase in preparation of functional oligosaccharide: CN. CN106367458A[P]. 2017-02-01
[17] Zhong C, Dui? B, Bolivar JM, Nidetzky B. Three-enzyme phosphorylase cascade immobilized on solid support for biocatalytic synthesis of cello-oligosaccharides[J]. ChemCatChem, 2020, 12(5): 1350-1358. DOI:10.1002/cctc.201901964
[18] Muschiol J, Peters C, Oberleitner N, Mihovilovic MD, Bornscheuer UT, Rudroff F. Cascade catalysis: strategies and challenges en route to preparative synthetic biology[J]. Chemical Communications: Cambridge, England, 2015, 51(27): 5798-5811. DOI:10.1039/C4CC08752F
[19] Li WQ, McArthur JB, Chen X. Strategies for chemoenzymatic synthesis of carbohydrates[J]. Carbohydrate Research, 2019, 472: 86-97. DOI:10.1016/j.carres.2018.11.014
[20] Seo DH, Jung JH, Lee JE, Jeon EJ, Kim W, Park CS. Biotechnological production of arbutins (α- and β-arbutins), skin-lightening agents, and their derivatives[J]. Applied Microbiology and Biotechnology, 2012, 95(6): 1417-1425. DOI:10.1007/s00253-012-4297-4
[21] Jurica K, Gobin I, Kremer D, ?epo DV, Grubeši? RJ, Kara?onji IB, Kosalec I. Arbutin and its metabolite hydroquinone as the main factors in the antimicrobial effect of strawberry tree (Arbutus unedo L.) leaves[J]. Journal of Herbal Medicine, 2017, 8: 17-23. DOI:10.1016/j.hermed.2017.03.006
[22] Oliveira I, Coelho V, Baltasar R, Pereira JA, Baptista P. Scavenging capacity of strawberry tree (Arbutus unedo L.) leaves on free radicals[J]. Food and Chemical Toxicology, 2009, 47(7): 1507-1511. DOI:10.1016/j.fct.2009.03.042
[23] Avonto C, Wang YH, Avula B, Wang M, Rua D, Khan IA. Comparative studies on the chemical and enzymatic stability of alpha- and beta-arbutin[J]. International Journal of Cosmetic Science, 2016, 38(2): 187-193. DOI:10.1111/ics.12275
[24] Li XY, Xia YY, Shen W, Yang HQ, Cao Y, Chen XZ. Characterization of a sucrose phosphorylase from Leuconostoc mesenterides for the synthesis of α-arbutin[J]. Chinese Journal of Biotechnology, 2020, 36(8): 1546-1555.
[25] Shen Y, LüXQ, Lin L, Li JH, Du GC, Liu L. Semi-rational design of sucrose phosphorylase and optimization of conditions for α-arbutin production[J]. Food and Fermentation Industries, 2020, 46(13): 1-9.
[26] Yu SH, Wang YC, Tian YQ, Xu W, Bai YX, Zhang T, Mu WM. Highly efficient biosynthesis of α-arbutin from hydroquinone by an amylosucrase from Cellulomonas carboniz[J]. Process Biochemistry, 2018, 68: 93-99. DOI:10.1016/j.procbio.2018.02.012
[27] Peng Y, Tang SY, Zhang Y, Zhu L, Ling JY, Hu MR, Tao Y. High-yield production of α-arbutin by an Escherichia coli whole-cell biocatalyst expressing amylosucrase[A]// National Symposium on Enzyme Engineering and Glycobiotechnology[C]. 2015
[28] Seo ES, Kang J, Lee JH, Kim GE, Kim GJ, Kim D. Synthesis and characterization of hydroquinone glucoside using Leuconostoc mesenteroides dextransucrase[J]. Enzyme and Microbial Technology, 2009, 45(5): 355-360. DOI:10.1016/j.enzmictec.2009.07.011
[29] Su GZ, Wang CG, Xue HY, Shi LQ. Sucrose phosphorylase mutant and its application in the production of glycosylglycerol: CN. CN107858335A[P]. 2018-03-30
[30] Takenaka F, Uchiyama H. Synthesis of α-D-glucosylglycerol by α-glucosidase and some of its characteristics[J]. Bioscience, Biotechnology, and Biochemistry, 2000, 64(9): 1821-1826. DOI:10.1271/bbb.64.1821
[31] Goedl C, Sawangwan T, Mueller M, Schwarz A, Nidetzky B. A high-yielding biocatalytic process for the production of 2-O-(alpha-D-glucopyranosyl)-sn-glycerol, a natural osmolyte and useful moisturizing ingredient[J]. Angewandte Chemie: International Ed in English, 2008, 47(52): 10086-10089. DOI:10.1002/anie.200803562
[32] Bolivar JM, Luley-Goedl C, Leitner E, Sawangwan T, Nidetzky B. Production of glucosyl glycerol by immobilized sucrose phosphorylase: options for enzyme fixation on a solid support and application in microscale flow format[J]. Journal of Biotechnology, 2017, 257: 131-138. DOI:10.1016/j.jbiotec.2017.01.019
[33] Duan PF, You JJ, Xu MJ, Yang TW, Shao ML, Zhang X, Rao ZM. Whole-cell biosynthesis of 2-O-α-D-glu- copyranosyl-sn-glycerol by recombinant Bacillus subtilis[J]. Chinese Journal of Biotechnology, 2020, 36(9): 1918-1928.
[34] Sugimoto K, Nomura K, Nishiura H, Ohdan K, Ohdan K, Hayashi H, Kuriki T. Novel transglucosylating reaction of sucrose phosphorylase to carboxylic compounds such as benzoic acid[J]. Journal of Bioscience and Bioengineering, 2007, 104(1): 22-29. DOI:10.1263/jbb.104.22
[35] Li Y, Li Z, He XY, Chen LL, Cheng YC, Jia HH, Yan M, Chen KQ. Characterisation of a Thermobacillus sucrose phosphorylase and its utility in enzymatic synthesis of 2-O-α-D-glucopyranosyl-L-ascorbic acid[J]. Journal of Biotechnology, 2019, 305: 27-34. DOI:10.1016/j.jbiotec.2019.08.018
[36] Gudiminchi RK, Nidetzky B. Walking a fine line with sucrose phosphorylase: efficient single-step biocatalytic production of l-ascorbic acid 2-glucoside from sucrose[J]. Chembiochem, 2017, 18(14): 1387-1390. DOI:10.1002/cbic.201700215
[37] Nakajima N, Ishihara K, Hamada H. Functional glucosylation of kojic acid and daidzein with the Eucalyptus membrane-associated UDP-glucosyltransferase reaction system[J]. Journal of Bioscience and Bioengineering, 2001, 92(5): 469-471. DOI:10.1016/S1389-1723(01)80298-X
[38] Vestergaard M, Ingmer H. Antibacterial and antifungal properties of resveratrol[J]. International Journal of Antimicrobial Agents, 2019, 53(6): 716-723. DOI:10.1016/j.ijantimicag.2019.02.015
[39] Sáez-Sáez J, Wang GK, Marella ER, Sudarsan S, Cernuda Pastor M, Borodina I. Engineering the oleaginous yeast Yarrowia lipolytica for high-level resveratrol production[J]. Metabolic Engineering, 2020, 62: 51-61. DOI:10.1016/j.ymben.2020.08.009
[40] Turner RS, Thomas RG, Craft S, Van Dyck CH, Mintzer J, Reynolds BA, Brewer JB, Rissman RA, Raman R, Aisen PS, et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease[J]. Neurology, 2015, 85(16): 1383-1391. DOI:10.1212/WNL.0000000000002035
[41] Kraus M, Grimm C, Seibel J. Redesign of the active site of sucrose phosphorylase through a clash-induced cascade of loop shifts[J]. Chembiochem, 2016, 17(1): 33-36. DOI:10.1002/cbic.201500514
[42] Gantt RW, Peltier-Pain P, Thorson JS. Enzymatic methods for glycol (diversification/randomization) of drugs and small molecules[J]. Natural Product Reports, 2011, 28(11): 1811-1853. DOI:10.1039/c1np00045d
[43] Kraus M, Grimm C, Seibel J. Reversibility of a point mutation induced domain shift: expanding the conformational space of a sucrose phosphorylase[J]. Scientific Reports, 2018, 8(1): 10490. DOI:10.1038/s41598-018-28802-2
 |
 |
 |
| 官网:www.cxbio.com | 微信服务号:iseebio | 微博:seebiobiotech |
 |
 |
 |
| 商城:mall.seebio.cn | 微信订阅号:seebiotech | 泉养堂:www.canmedo.com |
相关资讯
- 芦笋皂苷
- 全基因合成服务
- Nature发现破解神经退行性疾病的密码
- 新型抗菌药物——聚碳酸酯分子
- Nature:RAS突变激活胰腺癌细胞的巨胞饮作用,使得它们避免挨饿
- 乙炔树突聚合物 Acetylene dendrons
- 凝血酶(牛)Thrombin from bovine plasma
- 可用于破骨细胞抗酒石酸酸性磷酸酶 TRAP 染色试剂盒 TRAP Staining Kit
- SCREEN-WELL(R) 肾毒理化合物库 SCREEN-WELL(R) Nephrotoxicity library
- 西宝稳定性同位素 - 多种标记化合物 品专质优
新进产品
同类文章排行
- “乳源明星”酪蛋白酸钠:食品、日化、医药三大领域应用全景图
- 如何破解细胞培养中支原体污染难题
- 总及游离甲状腺素(TT4及FT4)-磁微粒化学发光法(AE/AP)解决方案(双抗体夹心法)
- CAR-T全链条关键产品及服务解决方案
- 超分子弹性蛋白:肌肤弹性的“隐形守护者”
- PDRN:不止护肤!新拓食品用途,双域焕新护健康
- 维生素K2:中国智造撬动全球千亿蓝海——从细胞抗衰到合成生物学的产业跃迁
- 细胞蛋白提取工具酶速查手册
- 抗缪勒管激素(AMH)-磁微粒化学发光法(AE/AP) +荧光免疫层析解决方案
- 2,5-呋喃二甲酸(FDCA):从生物质平台分子到绿色材料的革命
资讯文章
您的浏览历史




![[6,6]-苯基 C71 丁酸甲酯 [6,6]-苯基 C71 丁酸甲酯](http://www.cxbio.com/UploadFiles/Product/609771-63-3.gif)


